 A spectral power distribution (SPD) is a characterization of the power output of a particular light source for all the wavelengths of the visible spectrum. It maps the energy present at each wavelength, typically between wavelengths of about 380nm and 760 nm. The data required to create an SPD may be collected using a spectrophotometer. Power distributions of this type serve as a basis for the quantitative analysis of colour. The luminance and chromaticity of a colour can be derived from the SPD data, and the colour can then be described precisely within the CIE system. The CIE system characterizes colour using a luminance parameter known as Y, and two colour co-ordinates known as x and y which define a particular colour's position on the CIE chromaticity diagram. The CIE chromaticity diagram incorporates all the colours perceived by a normal human eye.
A spectral power distribution (SPD) is a characterization of the power output of a particular light source for all the wavelengths of the visible spectrum. It maps the energy present at each wavelength, typically between wavelengths of about 380nm and 760 nm. The data required to create an SPD may be collected using a spectrophotometer. Power distributions of this type serve as a basis for the quantitative analysis of colour. The luminance and chromaticity of a colour can be derived from the SPD data, and the colour can then be described precisely within the CIE system. The CIE system characterizes colour using a luminance parameter known as Y, and two colour co-ordinates known as x and y which define a particular colour's position on the CIE chromaticity diagram. The CIE chromaticity diagram incorporates all the colours perceived by a normal human eye.
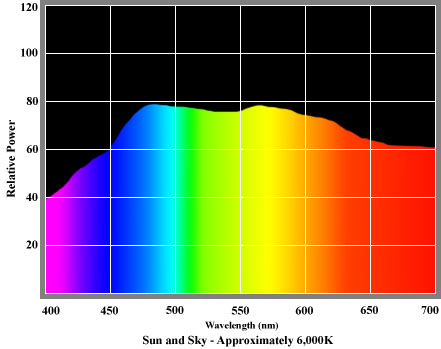
An SPD diagram for normal daylight reveals that this most natural of sources contains a comparatively uniform distribution of energy across the whole visible spectrum, and therefore provides excellent colour rendition. A typical SPD for daylight is shown on the left.
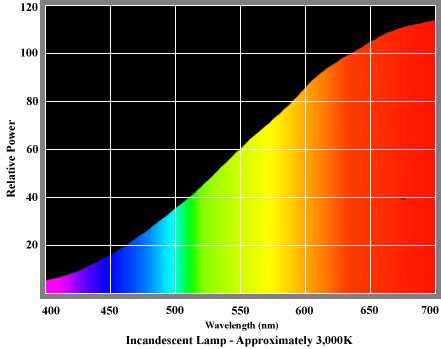 Most artificial light souces lack energy in the blue and green areas of the spectrum. In the case of incandescent sources, such as those traditionally used for domestic lighting, light is produced by heating a filament manufactured from a solid material until it radiates light. In very broad terms, this is similar to the way in which the Sun produces daylight, so it is not surprising that both daylight and incandescent sources have smooth spectral distributions. However, colours are rendered quite differently by incandescent sources. Most of the energy radiated by standard incandescent lamps is found in the longer wavelengths at the red end of the spectrum. Much less energy is radiated in the shorter wavelengths of the blues and greens. Blues are therefore not rendered very well.
Most artificial light souces lack energy in the blue and green areas of the spectrum. In the case of incandescent sources, such as those traditionally used for domestic lighting, light is produced by heating a filament manufactured from a solid material until it radiates light. In very broad terms, this is similar to the way in which the Sun produces daylight, so it is not surprising that both daylight and incandescent sources have smooth spectral distributions. However, colours are rendered quite differently by incandescent sources. Most of the energy radiated by standard incandescent lamps is found in the longer wavelengths at the red end of the spectrum. Much less energy is radiated in the shorter wavelengths of the blues and greens. Blues are therefore not rendered very well.
A fluorescent lamp uses gaseous discharge technology to produce light. An electric arc is generated between tungsten cathodes within a cylindrical glass tube filled with a low-pressure mercury vapour combined with various other gases. The arc is used to excite the mercury vapour to a point where it begins to generate radiant energy. This energy is produced largely at ultraviolet wavelengths. The glass tube containing the vapour is coated with one of several different phosphor-based materials, which then fluoresces to transform energy output at ultraviolet wavelengths to visible light.
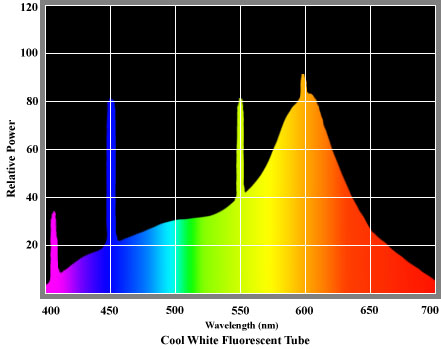 Phosphor-coated fluorescent tubes offer more color options than any other type of lamp. The chemical composition of the phosphor coating allows precise control of output in the red, green and blue wavelengths, enabling a variety of "warm" and "cold" colour temperatures to be generated. Some of these tubes provide excellent rendition of virtually all colors of the visible spectrum. A characteristic of this type of lamp is that spikes of energy are emitted at particular wavelengths, so their spectral power distributions are not as smooth as those of incandescent sources. A typical SPD for a cool fluorescent tube is shown on the right.
Phosphor-coated fluorescent tubes offer more color options than any other type of lamp. The chemical composition of the phosphor coating allows precise control of output in the red, green and blue wavelengths, enabling a variety of "warm" and "cold" colour temperatures to be generated. Some of these tubes provide excellent rendition of virtually all colors of the visible spectrum. A characteristic of this type of lamp is that spikes of energy are emitted at particular wavelengths, so their spectral power distributions are not as smooth as those of incandescent sources. A typical SPD for a cool fluorescent tube is shown on the right.






